Published On: 21st March, 2024
Abstract
Plants are essential to life on earth. Humans depend on plants for food, medicine, industrial development, and aesthetic use. The objective of this study is to establish somatic embryos from calli for the production of artificial seeds and to study the comparative characterization of plantlets from artificial seeds and natural seeds. Callus induction of Carica papaya L. using different explants was done. Callus induction varied with the explants. Somatic embryos were obtained from 5 to 7-week-old callus cultures. The zygotic embryos of papaya were excised and used for the production of artificial seeds by encapsulating them with sodium alginate in different concentrations (2% and 4%). A pot assay of these artificial seeds was conducted. Extract from leaves of plantlets grown from artificial seeds were used for chlorophyll estimation, and protein and carbohydrate quantification.
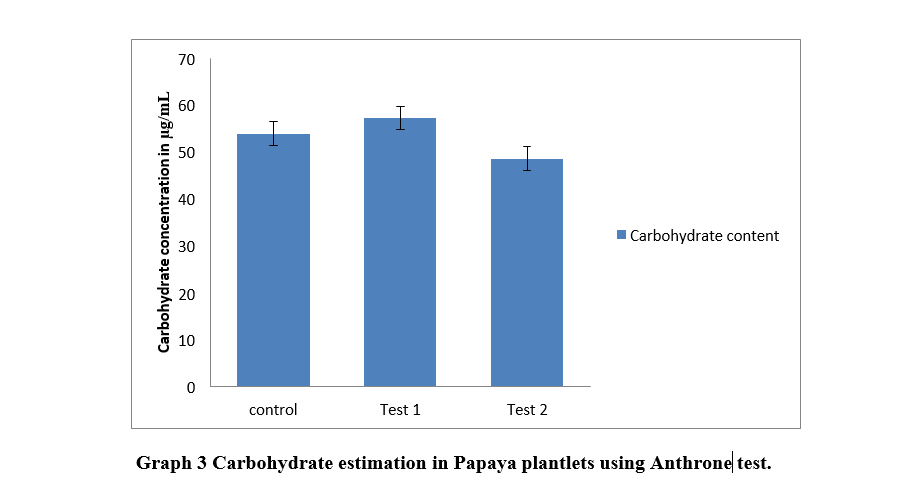
Artificial seeds of the zygotic embryo of papaya encapsulated in 4% sodium alginate excelled in almost all tests in comparison with artificial seeds with 2% sodium alginate encapsulation. Maximum chlorophyll content was of plants from 4% encapsulated embryo with 8.6 mg/ gm of tissue, whereas it was 2.9 mg/gm of tissue and 3.6 mg/ gm of tissue in plants from 2% encapsulated embryo and control respectively. The carbohydrate content was maximum in plants from 4% encapsulated embryo with 61 µg/ ml. The carbohydrate content in plants from 2% encapsulated embryo and natural seed was 56µg/ ml and 54µg/ ml respectively. Similarly, protein content in plants from 4% encapsulated embryo, 2% encapsulated embryo, and natural seeds was 240 µg/ml, 268 µg/ml, and 94 µg/ml respectively.
Key terminologies: Callus, Somatic embryos, Artificial seeds, Sodium alginate.
Introduction
Plants are considered renewable natural sources. Mankind has exploited plants for their own benefit. Human cultivation of plants is part of agriculture, which is the basis of human civilization. Plants provide sustenance for humans, either directly or through feeding domestic animals. The main source of organic compounds for industrial synthesis of a wide range of organic chemicals as well as for their physiological and therapeutic effects is medicinal plants. Plants can produce non-food items such as essential oils, waxes, resins, tannins, alkaloids, natural colors, pigments, and cork. In addition to being grown for their aesthetic value, thousands of plant species are also grown for their ability to block wind, regulate temperature, give shade, minimize noise, and stop soil erosion.
Using contemporary plant tissue culture techniques, micropropagation is the process of quickly growing stock plant material to generate a large number of offspring plants. Plants that have been genetically modified or grown using traditional plant breeding techniques can be multiplied using micropropagation. The conventional propagation practice for clonal propagation of plants is time-consuming and labor-intensive. Micropropagation, popularly known for large-scale clonal propagation, is a major and widely accepted practical application of plant biotechnology.
Advantages of in vitro micropropagation over conventional methods :
- Micropropagation enables growers to increase the production of plants that normally propagate very slowly such as Narcissus and other bulbous crops. The introduction of disease-free new cultivars is possible through micropropagation. One of the Rapid methods for cloning disease-free trees( Mishra et., 2010).
- Clonal mass propagation – an extremely large number of plants can be Through micropropagation, one can receive more than 1,000,000 plants annually from a single starting explant, as opposed to the 10,000 plants that can be obtained from an initial cutting in vegetative propagation.
- Culture is initialized from small parts of plants – so no need for much space: from 1 m to 2 m space in the culture room, 20000 – 100000 plants can be produced per
- Production of disease and virus-free This leads to simplification of the international exchange of plants.
- Vegetative propagation of sterile hybrids can be used as parent plants for seed E.g. Cabbage.
- In vitro cultures can be stored for a long time through 7] Breeding cycle can be shortened.
Plant tissue culture holds the key to accelerating food production and medicines by continuous production and supply of plantlets of the desired variety thereby reducing the land area. However, commercialization of such technology has been hampered by high production costs. Therefore, low-cost tissue culture technology is the need of the day. Plant growth regulators act as one of the chief components of plant tissue culture media and they are also expensive. The use of plant extracts as an alternative to these plant growth regulators can reduce the unit cost of plant production without compromising the quality of the plant.
Carica papaya L.
The papaya also known as pawpaw is a member of the Caricaceae family. Its scientific name is Carica papaya L. First domesticated in Southern Mexico and Central America, now also available in various states of India including Tamil Nadu, Karnataka, Andhra Pradesh, and Gujarat.
Classification
Kingdom: Plantae
Division: Magnoliophyta Class: Magnoliopsida
Order : Brassicales
Family : Caricaeae
Genus: Carica
Species: Papaya L.
Vernacular name: Hindi – Papeeta

Fig. 1 Carica papaya
L.
Morphology
Papaya is herbaceous perennial plant. It has single erect stem with height 8 to 10 meters. The bark is light brown to light green, smooth, with horizontal ridges from previous years’ leaf scars. It has alternate leaf arrangement, simple leaf type, parted leaf margin, star shaped leaves, palmate type of leaf venation, and green to olive green leaf colour with 18 to 23 inches leaf blade length. Male flowers emerge in branched clusters on 1 to 2 inch long stalks; female flowers emerge singly or in clusters at leaf axils. Papaya fruit is oblong or pear shaped and has the length of 3 to 15 inches. Fruit turns from green to yellow orange when ripe.
Culture condition
Papaya is the fast growing crop as compared to other perennials. It takes about 4 to 5 months to flower after germination of seeds and bear fruits 8 months after it. Annually papaya tree can produce 35 to 40 fruits, with each fruit weighing almost 1 to 2 kgs. The ideal temperature for papaya to grow is 21ºC. Papaya crop flowers best in bright sunlight in soil with moisture.
Traditional use and nutritional value
Papaya effectively cures abdominal infections. It also works as medicine against dyspepsia, hyper acidity, dysentery and constipation. Papaya is considered as healthy to our immune system by increasing resistance to cough and cold. Papaya is favoured worldwide for consumption, in its natural form as well as in processed forms like jam, pulp and sweets (Aravind et. al., 2013). Other parts of plant like leaves and seeds are used as additives in flours and teas. Papaya fruit is low in calories and sodium but has high amount of dietary fibres, vitamin A and C. Papaya produces an enzyme called papain which is popularly used in hair and face care products. This enzyme also has myriad applications in meat industry, leather industry and beer industry. Phenolic compounds like alpha tocopherol and beta tocopherol, beta carotene, carotenoids and glucosinolates are present in papaya seeds. Leaves of papaya also contain proanthocyanins, flavonoids and saponins( Bindu et. al., 2017).
Problem
Plant growers get to know the gender of plants only after plant flowers and bear fruit that takes 4 to 5 months. As papaya has a unisexual flower and only females can bear fruit. In ideal situation farmers need majority of plants to be female and a few male plants for the purpose of pollination as an effective strategy to utilize land potential to its maximum. The rate of germination without external assistance is low. Without fertilizers their germination can take up to five weeks.
Materials and Methodology
Sample collection
Papaya sample was collected from Alpha nursery, Gangapur, Nashik. Two types of explants of papaya were chosen for study viz., immature zygotic embryos excised from unripe fruit and leaf.
Callus induction and Aseptic transfer
The samples were washed in running tap water to avoid desiccation and then rinsed with Tween 20 and washed with autoclave distilled water 5 times. Surface sterilization was done under aseptic conditions in laminar air flow cabinet. They were surface sterilized with 70% ethyl alcohol (1 minutes) followed by 0.1 % HgCl2 for 2 minutes.
Leaf segments from papaya leaf explant was excised directly and cultured on MS medium supplemented with NAA and Dichlorophenoxyacetic acid (2,4-D).
The explants in all experiment were maintained at 25±2°c in a constant temperature growth room, under light intensity of 2000 lux for a 16/8 hours photoperiod. After 4 week of inoculation, the culture response was recorded.
Subculture
The whole callus mass was taken out aseptically on a sterile petridish and was divided into several small pieces using flame sterilized scalpel and forceps. Each piece of callus tissue was transferred to a culture bottle containing fresh MS medium fortified with plant growth promoter as given in table 3.1.
The explants in all experiment were maintained at 25 ± 2°C in a constant temperature growth room, under cool white fluorescent light using a 16 hours photoperiod. After 4 week of inoculation, the culture response was recorded.
Somatic embryogenesis
Murashige and skoog (MS) medium, containing 3% sucrose and agar (8g/L) was used throughout the experiments. The pH of media was adjusted to 5.7 pH. The medium was then autoclave at 121°C for 25 minutes.
Somatic embryo induction from callus cells was done using plant growth promoter in the concentration shown below,
Table 1 : Plant growth regulator treatment in somatic embryo induction.
|
Explants |
Treatment |
|
|
Papaya |
Leaf |
0.02mg/L NAA + 0.5mg/L BAP |
Synthetic seed production
All pre-embryonic calli from leaf explant of Papaya( Kumari et. al., 2018).
The explants in all experiments were maintained at 25 ± 2 ºC in a constant temperature growth room, under 2000 lux light intensity using a 16/8 hour photoperiod.
Pot Assay and Statistical Analysis
Encapsulated zygotic embryo’s were sown in soil for pot trials. Normal seeds were also sown as control for comparative analysis.
Visual observations of the cultures were made after every alternate day and data was taken as defined in each experiment. The data was analysed using statistical methods. Growth index and vigour index was also calculated. Chlorophyll estimation, protein content and carbohydrate quantification was performed. Statistical analysis using one way ANOVA was performed.
Chlorophyll estimation from Papaya samples from pot trials. (Kamble et. al., 2015)
Method
- Take 1 gm of leaf explant from every sample, and crush it using mortar and pestle using 80% acetone.
- Aseptically transfer 1 ml of papaya leaf extract into centrifuge 3] Make the final volume 5 ml using 80% acetone.
- Centrifuge it at 7000 rpm for 10 min.
- Measure the absorption at 663 nm and 645 nm using 80% acetone as reference solution.
Formulae
Milligram of chlorophyll a / gm tissue = (12.7×A663 – 2.69×A645)×V
1000 × W
Milligram of chlorophyll b / gm tissue = (22.9 × A645 – 4.68 × A663 )× V
1000 × W
Total chlorophyll in milligram / gm tissue = (20.2 × A645 + 8.02 × A663 ) × V
1000 × W
Where, A = absorption at particular wavelength V = final volume of acetone
W = weight of sample
Estimation of protein contents of Papaya samples by Folin-Lowry method.(Waterborg et. al., 1996)
Materials
1. Alkaline sodium carbonate solution ( 2 of sodium carbonate dissolved in 0.1 N sodium hydroxide and volume is made up to 100 ml with distilled water).
2. Copper sulfate solution (0.5 gm copper sulfate dissolved in 1% solution of potassium sodium tartarate and volume is made up to 100 ml with distilled )
3. Alkaline copper solution (50 ml of solution 1 and 1ml of solution 2)
4. Folin ciocalteau reagent
5. Standard BSA stock solution of 200 µg/ml in distilled water.
Method
- 1 ml of various dilution of protein solution were pipetted out in clean dry test tubes .
5 ml alkaline copper solution is added in each tube.
- The contents of tubes were mixed and allow to stand at room temperature for 10 minutes.
- After this 5 ml of FC reagent was added rapidly with continuous mixing allowed to stand at room temperature for 30 minutes.
- The absorbance then read at 650 nm against blank.
- The papaya leaf extract was centrifuged at 2000 rpm for 2 min then the cells were crushed and used for further estimation.
Carbohydrate estimation by Anthrone method (Yemm et.al., 1954). Materials
Equipments – UV Spectrophotometer, Vortex mixer, Water bath
Reagents
- Anthrone reagent : Dissolve 2g of Anthrone in 1 litre of concentrated H2SO4. Use freshly prepared reagent for the assay.
- Glucose stock solution : 200 µg glucose per mL distilled water.
Procedure
- To each tube with 1mL papaya leaf extract , add 5 mL of anthrone reagent (supplied) and mix well by Cool the tubes.
- Keep the tubes in boiling water bath for 10
- Cool to room temperature and measures optical density at 620 nm against a blank .
Result
Almost 5 days after sowing zygotic embryos, papaya artificial seeds initiated germination. Among papaya artificial seeds zygotic embryos encapsulated in 4% sodium alginate showed better results as compared to zygotic embryos encapsulated in 2% sodium alginate. In case of 4% encapsulated embryos 40 out of 50 seeds germinated and in case of 2 % encapsulated embryos 30 out of 50 seeds germinated, where as only 20 out of 50 seeds were germinated (Fig 2 and Table 2).
The root and shoot length of 4% encapsulated embryos were found to be more than 2% encapsulated embryos of papaya. Number of leaves in 4 % encapsulated sample is more than rest of others (Table 2). Vigor index of 4% encapsulated embryos was greater than 2% encapsulated embryo which in turn was greater than control (Table 3).
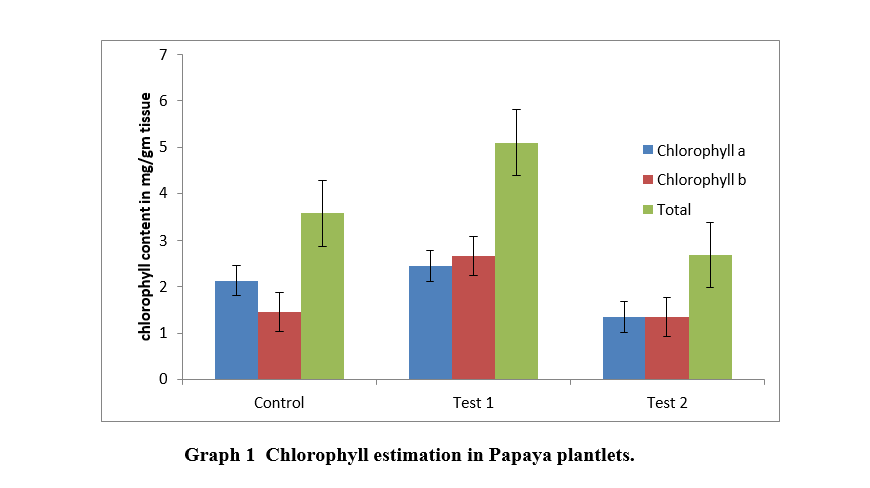
Chlorophyll a and b content of all test and control plantlet was calculated, and it was found that chlorophyll a and b in plantlets from 4% encapsulated artificial seeds ( Test 1) was more than plantlets from control (Table 4 and Graph 1). Chlorophyll a and b in plantlets from 2% encapsulated artificial seeds ( Test 2) was less than plantlets from control (Table 4 and Graph 1).
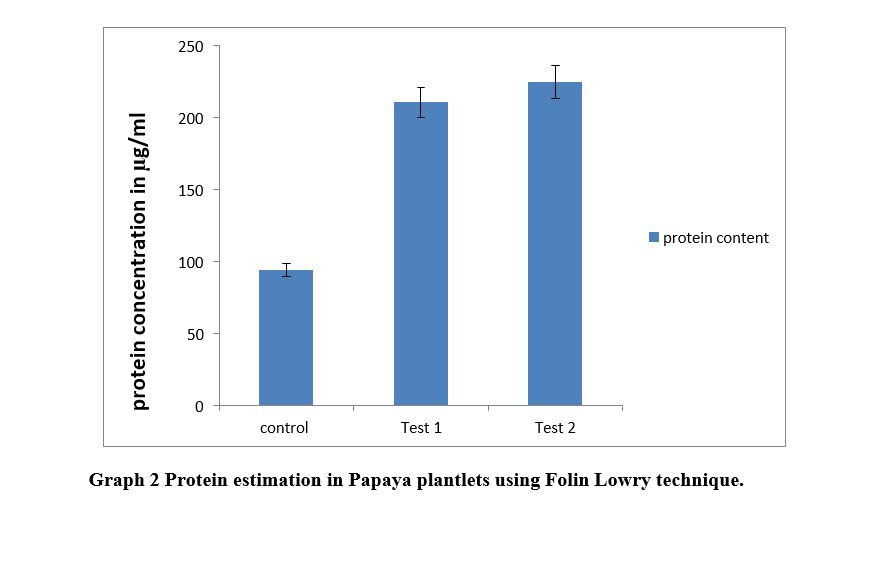
Protein estimation of all test samples and control was done. It was found that all test samples have significantly higher quantity of proteins than control (Graph 2).
In Anthrone test, carbohydrate content of 4% encapsulated artificial seeds was found to be more than 2% encapsulated embryo.
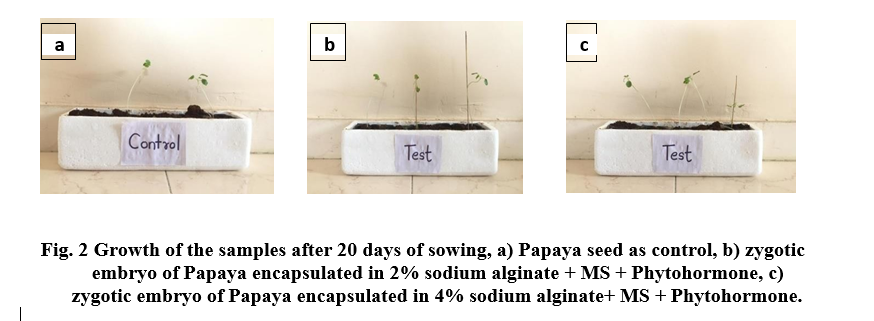

Table 2 Growth parameters of encapsulated zygotic embryo of Papaya and Drumstick after 20 days .
|
Explants |
Total no. of seeds |
%Seed germination |
Average No. of Leaves/ Plantlet |
Average Shoot length/ Plantlet (cm) |
Average Root length/ Plantlet (cm) |
|
Papaya control |
50 |
40 |
6 |
10.6 |
2.35 |
|
2% encapsulated embryo |
50 |
60 |
7 |
12.6 |
2.51 |
|
4% encapsulated embryo |
50 |
80 |
13 |
13.8 |
2.69 |
Table 3 Vigor index of papaya germination.
|
Explants |
Vigor index |
|
Papaya control |
518 |
|
2% encapsulated papaya embryo |
906 |
|
4%encapsulated papaya embryo |
1319 |
Table 4 Chlorophyll content in Papaya plantlets.
|
|
Chlorophyll a (mg / gm of tissue) |
Chlorophyll b (mg / gm of tissue) |
Total Chlorophyll (mg / gm of tissue) |
|
Control |
2.123 |
1.451 |
3.576 |
|
Test 1 |
2.441 |
2.656 |
5.096 |
|
Test 2 |
1.345 |
1.33 |
2.681 |
*Test 1:2% encapsulated papaya embryo ; Test 2 : 4%encapsulated papaya embryo
One way ANOVA of control, artificial seeds encapsulated in 2% sodium alginate and artificial seeds encapsulated in 4% sodium alginate was performed. One way ANOVA for height of plantlets , chlorophyll content , protein content and carbohydrate content was calculated for significant differences in means estimation.
P-value for height of plantlets was 0.034527, which is ≤ 0.05. Thus the difference in mean is significant.
P-value for chlorophyll content was 0.318283 , which is ≥ 0.05. Thus the difference in mean is not significant.
P-value for protein content was 0.002647, which is ≤ 0.05. Thus the difference in mean is significant.
P-value for carbohydrate content was 0.193533 , which is ≥ 0.05. Thus the difference in mean is not significant.
Discussion and Conclusion
Difficulties in seed germination and viability has always been a major problem for farmers. Artificial seed production provides an alternative and solves this problem. In this study calli were induced from various explants of papaya. Different plant growth hormones were used for these plant sample to induce calli. These calli were subcultured on similar media to prevent them from desiccation and to provide them with fresh nutrients. These subcultured calli were then further stimulated to go for somatic embryogenesis. After 2 week’s incubation in 2.99mg/L BAP + 1.997mg/L Kinetin drumstick embryonic cells from maturation medium were harvested and visually observed under microscope. In present study, 5 cell stage and heart shaped stages of embryo development were observed. These completely matured somatic embryos can be used to produce artificial seeds.
Zygotic embryos from seeds of papaya were excised and used for synthesis of artificial seeds. These artificial seeds were produced using two different concentrations of sodium alginate i.e 2% and 4%, and their comparative study was conducted. Anand et. al., 2018 performed optimization of various concentration of sodium alginate in artificial seed production, where he found 2% and 4% had shown best results. These artificial seeds from zygotic embryos were sown in pots and were studied visually. Their chlorophyll content, protein and carbohydrate concentration was estimated. Maximum chlorophyll content in this study was of plant from 4% encapsulated embryo with 8.664 mg/ gm of tissue , whereas it was 2.967 mg/gm of tissue in Kamble et. al,(2015). Protein estimation was done using Follin laury technique where maximum protein estimated was found in 2% encapsulated embryo of papaya (268 µg/mL ). Waterborg, Jakob & Matthews, Harry. (1996) estimated the same as 240 µg/mL. Carbohydrate content was quantified as 61 µg/ mL in Papaya artificial seed with 4% encapsulation. Yemm et. al. in 1954 estimated it to be 56 µg/ mL. Present study suggests an alternative technique for growing plant in an efficient way than convential method.
The artificial seeds produced had more chlorophyll a and b. Artificial seeds also had relatively high concentration of proteins and carbohydrates. The rate of growth of plantlets germinated from artificial seeds was relatively higher than normal seeds. Thus artificial seeds has potential to replace natural seeds.
Papaya that has problem of gender identification can used plant tissue culture technique for micropropagation, where farmers know the gender prior to cultivation.
References
Ali, A., Gull, I., Majid, A.B., Saleem, A., Naz, S., &Naveed, N.H. (2012).In vitro conservation and production of vigorous and desiccate tolerant synthetic seeds in Stevia rebaudiana. Journal of Medicinal Plants Research, 6.
Aravind, G. &Bhowmik, Debjit& S, Duraivel& Harish, Gudivada.(2013). Traditional and medicinal uses of Carica papaya.Journal ofMedicinal Plants Studies. 1. 7-15.
Bindu, B., &Podikunju, B. (2017). Tissue Culture Protocol for In-vitro Propagation of Papaya (Carica papaya L.). Journal of KrishiVigyan, 6, 205-212.
Chand, Suresh &Pandey, Ashu&Verma, Oshin. (2019). In vitro regeneration of Moringaoleifera Lam.: A medicinal tree of family Moringaceae. Indian Journal ofGenetics and Plant Breeding (The).79. 10.31742/IJGPB.79.3.10.
Devendra, B.N., Talluri, V.P., &Srinivas, N. (2012). Callus induction and somatic embryogenesis of MoringaoleiferaLam an anti-radiation plant. International Journal of Agricultural Technology, 8, 1953-1963.
Farzana, ARF &Palkadapala, PGVN &Meddegoda, KMMN &Samarajeewa, PK &Eeswara,
Janakie. (2008). Somatic embryogenesis in papaya ( Carica papaya L.) cv. Rathna. Journal of The National Science Foundation of Sri Lanka – J NATL SCI FOUND SRI LANKA. 36. 10.4038/jnsfsr.v36i1.132.
Kamble, Pramod&Giri, Sanjay & Mane, Ranjeet&Tiwana, Anupreet. (2015). Estimation of Chlorophyll Content in Young and Adult Leaves of Some Selected Plants. Universal journal of environmental researchand technology. 5. 306-310.
Keshvari, Tahereh&Najaphy, Abdollah&Kahrizi, Danial&Zebarjadi, Alireza.(2018). Callus induction and somatic embryogenesis in Stevia rebaudianaBertoni as a medicinal plant.Cellular and MolecularBiology. 64. 46. 10.14715/cmb/2018.64.2.9.
Knowles, Kaye &Dhir, S. &Dhir, Sarwan.(2004). Somatic embryogenesis and plant regeneration in Stevia rebaudiana.In Vitro Cellular & Developmental Biology – Animal. 40. 40A-40A.
Kumari, Swati &Trivedi, Mala &Shukla, Neelam& Mishra, Maneesh&Verma, Swati. (2018). POLYAMINE MEDIATED GENOTYPE INDEPENDENT SOMATIC EMBRYOGENESIS IN PAPAYA (CARICA PAPAYA L.). Plant Archives. 18. 581-589.
Mani, Arghya& Roy, Sourav.(2020). Nutritional importance and medicinal properties of Drumstick. 6. 52-53.
Meenakshi Banerjee &PriyankaSarkar(2010).Somatic embryogenesis in Stevia rebaudianaBertoni using different concentration of growth hormones.International Journal ofPlant Sciences, Vol. 5(1) : 284-289.
Mishra, Maneesh& Chandra, Ramesh &Pati, Rajesh & S, Jain.(2010). Shoot tip transformation in papaya (Carica papaya L.).ActaHorticulturae.851. 10.17660/ActaHortic.2010.851.32.
Naranjo EJ, Fernandez Betin O, Urrea Trujillo AI, Callejas Posada R, AtehortúaGarcés L. Effect of genotype on the in vitro regeneration of Stevia rebaudiana via somatic embryogenesis. Acta biol. Colomb. 2016;21(1):87-98.
Nower, Ahmed. (2014). In Vitro Propagation and Synthetic Seeds Production: An Efficient Methods for Stevia rebaudianaBertoni. SugarTech. 16. 10.1007/s12355-013-0228-7.
Rihan HZ, Kareem F, El-Mahrouk ME, Fuller MP. Artificial Seeds (Principle, Aspects and Applications). Agronomy. 2017; 7(4):71.
Ryavalad, Shivayogi&Malabasari, T.A. &Tirakannanavar, Shantappa&Uppar, D.S. &Biradar,
B.D. &Mantur, S.M.. (2019). Micropropagation Studies in Papaya (Carica papaya L.) cv. ‘Surya’.International Journal of Current Microbiology and Applied Sciences. 8. 2362-2367. 10.20546/ijcmas.2019.804.275.
Saha, Moitreyee. (2004). Generation of synthetic seeds from different varieties of Carica papaya L..Journal of Tissue ResearchISSN 0971- 2283. 4. 207-209.
Saini, R. K., Shetty, N. P., Giridhar, P., &Ravishankar, G. A. (2012). Rapid in vitro regeneration method for Moringaoleifera and performance evaluation of field grown nutritionally enriched tissue cultured plants. 3 Biotech, 2(3), 187–192.
Saxena, A., Shukla, M., Saxena, P. (2019). Synthetic Seeds: Relevance to Endangered Germplasm Conservation In Vitro. In: Faisal, M., Alatar, A. (eds) Synthetic Seeds .Springer, Cham.
Waterborg, Jakob& Matthews, Harry. (1996). The Lowry Method for Protein Quantitation. 10.1007/978-1-60327-259-9_2.
YEMM, E. W., & WILLIS, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. The Biochemical journal, 57(3), 508–514.